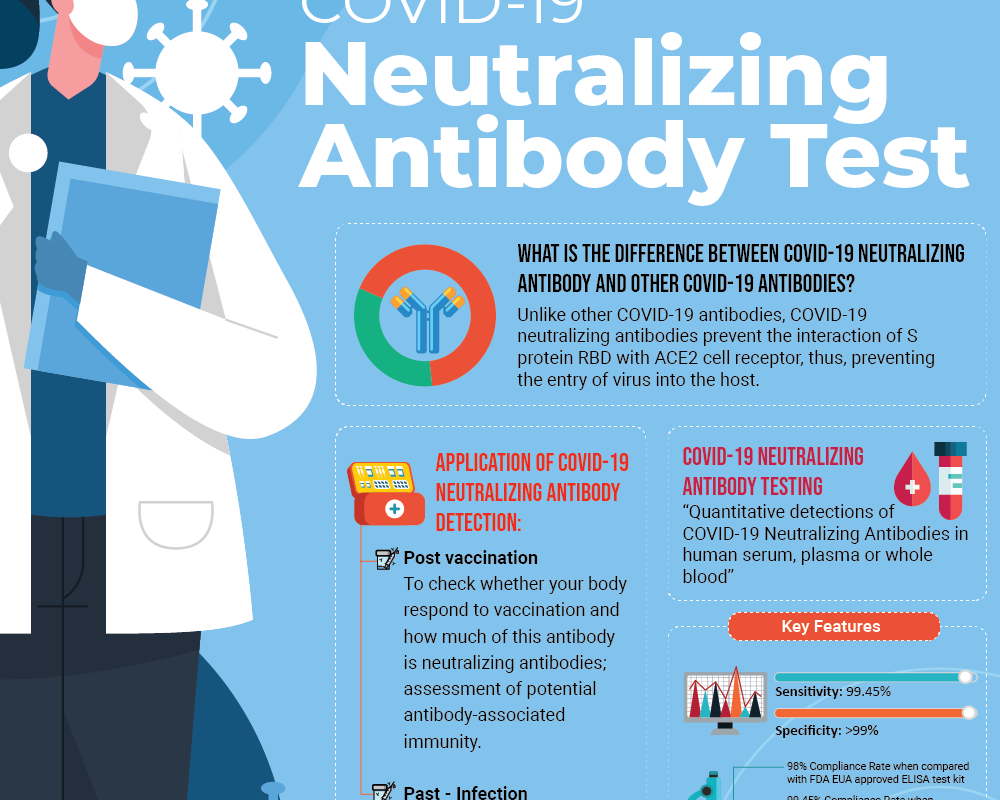
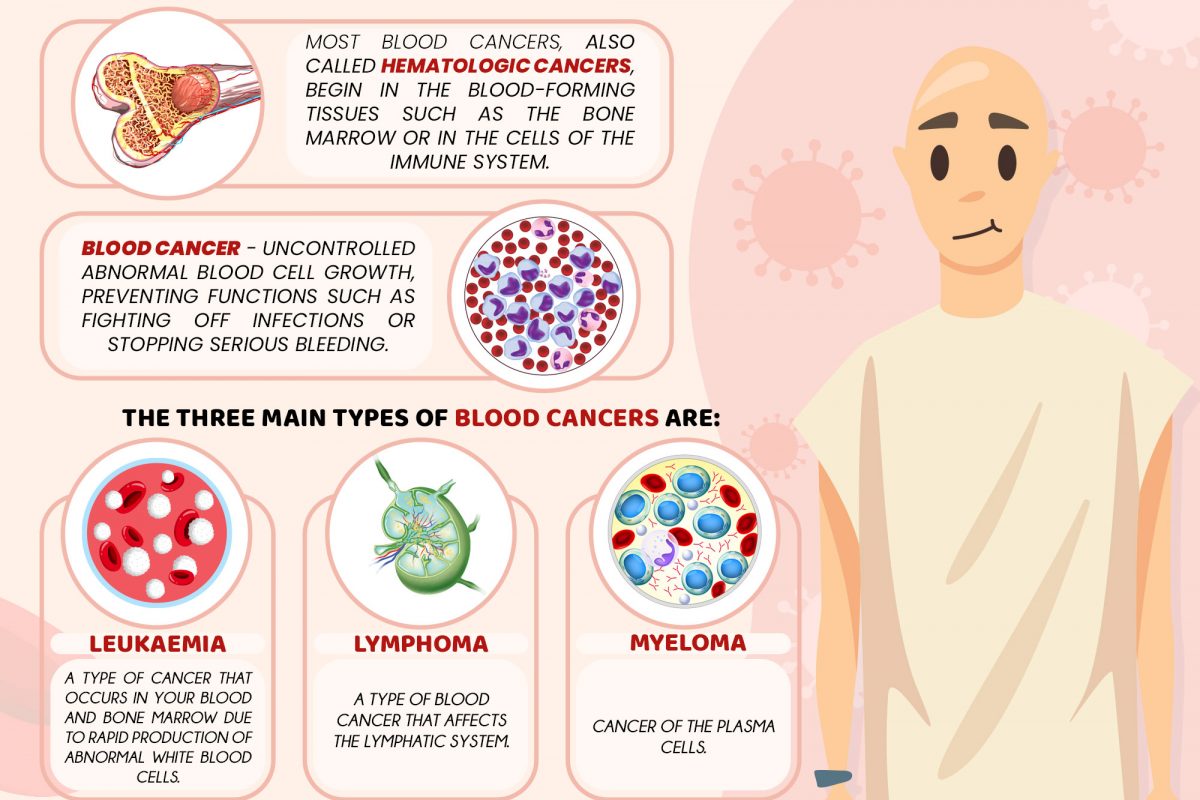
Increase the Success Rate of IVF via PGS
In Vitro Fertilization (IVF) is an assisted reproductive technology (ART) to achieve pregnancy, where the process of fertilization is manually performed in a laboratory dish by combining extracted eggs and retrieved sperms. Once the fertilized eggs have multiplied, the embryos are then transferred to the uterus for a pregnancy to begin.1, 2, 3 This procedure is commonly conducted in couples who have difficulty in achieving pregnancy through unprotected regular intercource.2
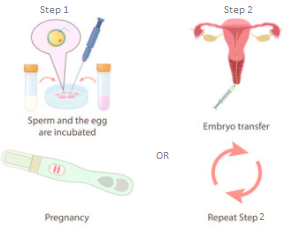
Figure 1: The process of In Vitro Fertilisation (IVF).4
Who needs IVF treatment?
IVF can be used to treat infertility in the following cases1:
- Unexplained infertility
- Women with ovulation disorders; premature ovarian failure, uterine fibroids
- Blocked or damaged fallopian tubes
- Male factor infertility; reduced sperm count or motility
- Individuals with genetic disorder
What is Preimplantation Genetic Screening (PGS)?
PGS is a specialised testing to screen whether cells from a cultured embryo contain any chromosomal abnormalities or balance number of chromosomes prior to implantation. PGS is highly recommended for patients with history of repeated unexplained miscarriages, recurrent IVF failures or couples with known abnormality in their chromosomal who worry passing it on to their offspring.
Common chromosomal abnormality PGS screens for are:
- Down Syndrome
- Patau Syndrome
- Edwards Syndrome
- Sex chromosomes abnormality
The process of PGS requires a small number of cells from the cultured embryos typically at day 5 (blastocyst stage) and cells are screened for normal number of chromosomes which is 46 (23 chromosomes inherited from each parents). If all chromosomes are intact with no abnormalities observed (called euploidy) then the embryo is suitable for implantation.6, 7, 10, 11, 12 Your fertility doctors will consult of the best embryos to implant in order to yield a successful pregnancy.
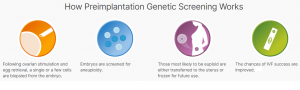
Figure 2: How Preimplantation Genetic Screening (PGS) Works.8
Reference
1- IVF – In Vitro Fertilization. (2019, April 24). American Pregnancy Association. https://americanpregnancy.org/getting-pregnant/infertility/in-vitro-fertilization-70966/
2- Brezina, P. R., & Zhao, Y. (2014). In Vitro Fertilization. ELSEVIER, 2014(3), 1–9. https://doi.org/10.1016/B978-0-12-801238-3.00262-2
3- Infertility FAQs. (n.d.). Division of Reproductive Health, National Center for Chronic Disease Prevention and Health Promotion. Retrieved April 8, 2021, from https://www.cdc.gov/reproductivehealth/infertility/index.htm
4- In Vitro Fertilisation. (2019, April). [Photograph]. Women’s Health Arm of Singapore Medical Group. https://smgwomenshealth.sg/news-articles/pgd-pgs-testing/
5- Harper, J. C., Geraedts, J., Borry, P., Cornel, M. C., Dondorp, W., Gianaroli, L., … & Macek, M. (2013). Current issues in medically assisted reproduction and genetics in Europe: research, clinical practice, ethics, legal issues and policy. European Journal of Human Genetics, 21(2), S1-S21.
6- De Rycke, M., & Sermon, K. (2010). Preimplantation Genetic Diagnosis. In Molecular Diagnostics (pp. 485-500). Academic Press.
7- Illumia. (2015). The STAR Trial [Brochure]. https://www.illumina.com/content/dam/illumina-marketing/documents/clinical/rgh/star-one-pager-web.pdf
8- Improving IVF outcomes with PGS. (n.d.). [Photograph]. Illumina. https://www.illumina.com/clinical/reproductive-genetic-health/preconception-fertility/pgs.html
9- Kimelman, D., Confino, R., Confino, E., Shulman, L. P., Zhang, J. X., & Pavone, M. E. (2018). Do patients who achieve pregnancy using IVF-PGS do the recommended genetic diagnostic testing in pregnancy?. Journal of assisted reproduction and genetics, 35(10), 1881–1885. https://doi.org/10.1007/s10815-018-1289-z
10- Improving IVF outcomes with PGS. (n.d.-b). Illumina. Retrieved April 9, 2021, from https://www.illumina.com/clinical/reproductive-genetic-health/preconception-fertility/pgs.html
11- Yang, Z., Liu, J., Collins, G. S., Salem, S. A., Liu, X., Lyle, S. S., Peck, A. C., Sills, E. S., & Salem, R. D. (2012). Selection of single blastocysts for fresh transfer via standard morphology assessment alone and with array CGH for good prognosis IVF patients: results from a randomized pilot study. Molecular cytogenetics, 5(1), 24. https://doi.org/10.1186/1755-8166-5-24