Testing for TB is critical prior to anti-TNF-α therapy
TNF-α inhibitor recipients face an increased risk of developing active TB
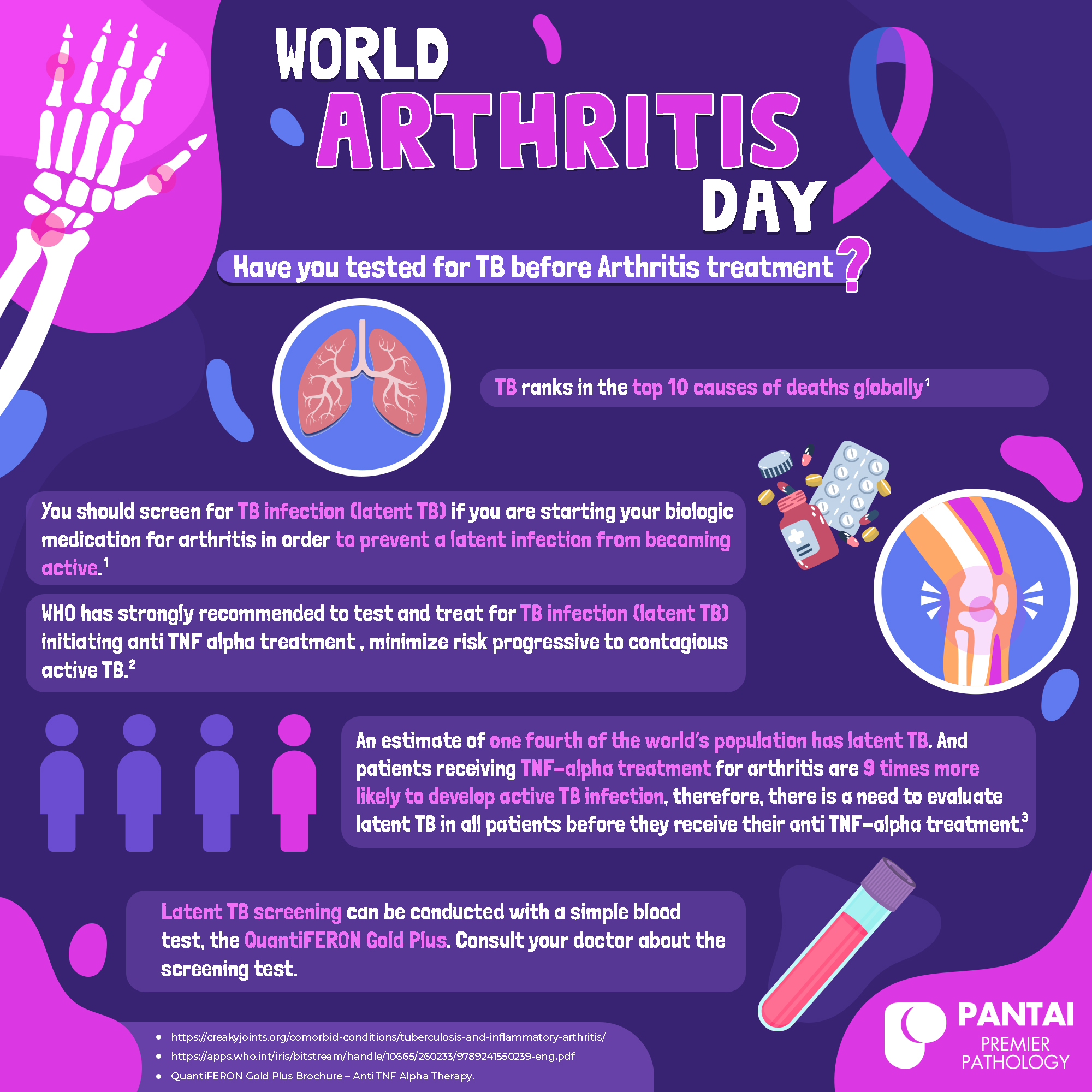
Autoimmune disorders such as rheumatoid arthritis, inflammatory bowel disease and Crohn’s disease are commonly treated with biologics to slow progression of the disease. Unfortunately, biologics such as TNF-α inhibitors can also increase the likelihood that patients carrying latent TB infection will progress to active TB (1–3). As a result, many biologic treatments carry a warning stating that TB infection should be investigated and treated prior to initiating therapy.
- More than one third of the world’s population is believed to carry latent TB infection (4)
- Patients receiving TNF-α inhibitor therapy face up to a 9-fold increased relative risk of developing active TB (5)
- TB reactivation risk should be evaluated in all patients prior to biologic therapy (2, 6)
Published data indicate that QuantiFERON technology may provide more accurate detection of TB infection prior to start anti-TNF-α treatment
Studies performed among patients with chronic immune diseases have reported improved performance by detecting TB infection with QuantiFERON technology compared to the tuberculin skin test (TST) (1, 2, 7, 8).
“In a TB-endemic population, the QuantiFeron-TB Gold In-Tube assay seemed to be a more accurate test for detection of LTBI in RA patients compared with the TST, and may potentially improve the targeting of prophylactic therapy before treatment with anti-TNF agents.” – Ponce de Leon (2008)
Before you initiate TNF-α inhibitor therapy, get tested with QuantiFERON-TB Gold Plus for accurate TB detection. For more information on the tests provided, please contact us at +603-42809115 (Customer Service) or email us at info@premierpathology.com.my
References:
- Matulis, G., Juni, P., Villiger, P.M., and Gadola, S.D. (2008) Detection of latent tuberculosis in immunosuppressed patients with autoimmune diseases: performance of a Mycobacterium tuberculosis antigen-specific interferon gamma assay. Ann. Rheum. Dis. 67, 84–90.
- Cantini, F., et al. (2017) Risk of tuberculosis reactivation in patients with Rheumatoid Arthritis, Ankylosing Spondylitis, and Psoriatic Arthritis receiving non-anti-TNF-targeted biologics. Mediators Inflamm. 2017:8909834
- Swaminath,A. Bhadelia, N., and Wang, Y.C. (2013) Cost-effectiveness of QuantiFERON testing before initiation of biological therapy in inflammatory bowel disease. Inflamm. Bowel Dis. 19, 2444–2449.
- World Health Organization. Tuberculosis Fact Sheet. http://www.who. int/mediacentre/factsheets/fs104/en/. Accessed Sept 18 2017.
- Lobue, P. and Menzies, D. (2010) Treatment of latent tuberculosis infection: An update. Respirology. 15, 603.
- World Health Organization. (2015) Guidelines on the management of latent tuberculosis infection. WHO/HTM/TB/2015.01.
- Ponce de Leon, D., et al. (2008) Comparison of an interferon-gamma assay with tuberculin skin testing for detection of tuberculosis (TB) infection in patients with rheumatoid arthritis in a TB-endemic population. J. Rheumatol. 35, 776–781.
- Mariette, X., et al. (2012) Influence of replacing tuberculin skin test with ex vivo interferon γ release assays on decision to administer prophylactic antituberculosis antibiotics before anti-TNF therapy. Ann. Rheum. Dis. 71, 1783–1790.