Liquid biopsy for detection of EGFR T790M mutation in Non-Small Cell Lung Cancer (NSCLC)
About Non-Small Cell Lung Cancer (NSCLC)
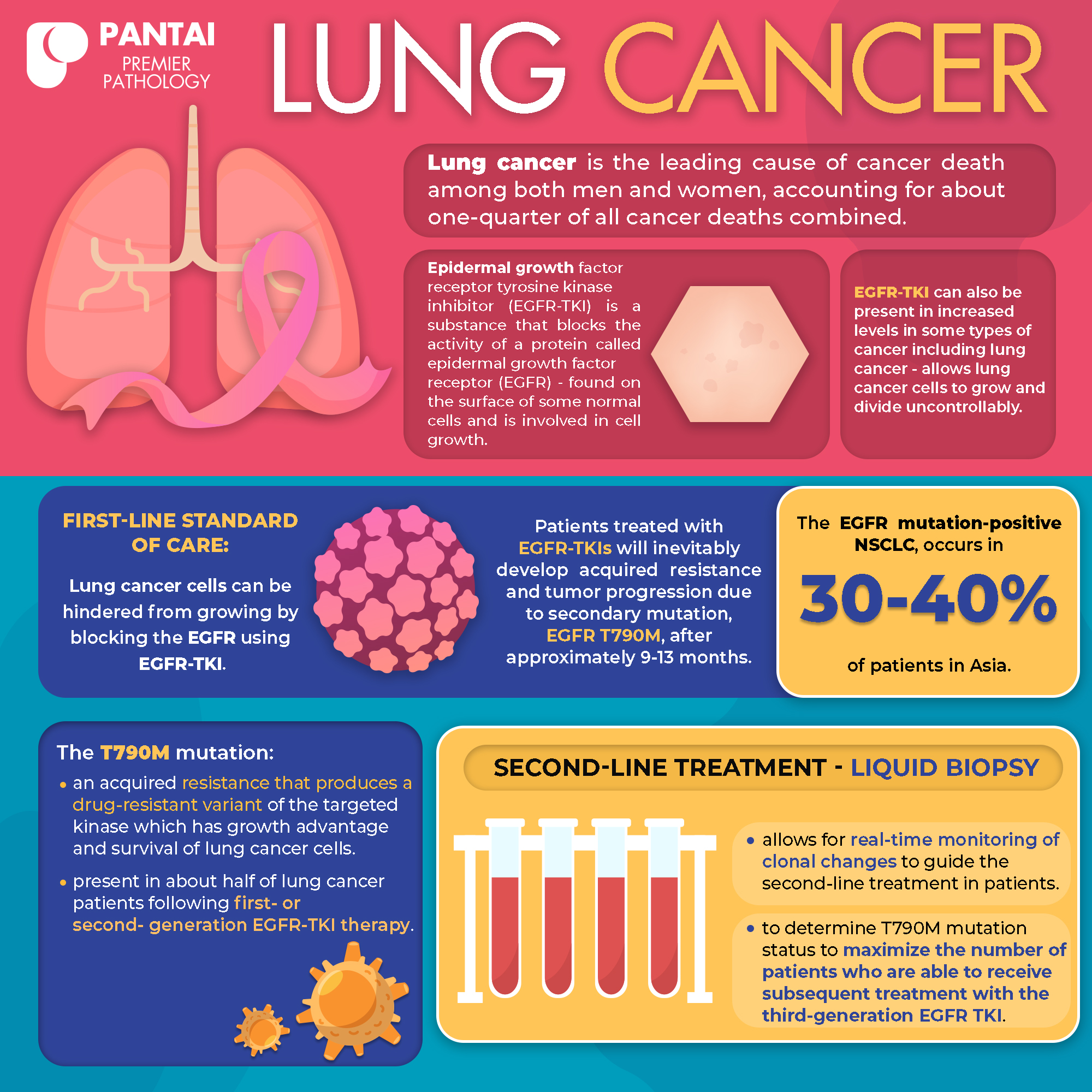
Lung cancer is the leading cause of cancer death among both men and women, accounting for about one-quarter of all cancer deaths combined.1
First-line Standard of Care
Epidermal growth factor receptor tyrosine kinase inhibitor (EGFR-TKI) is a substance that blocks the activity of a protein called epidermal growth factor receptor (EGFR), that is found on the surface of some normal cells and is involved in cell growth. This protein can also be present in increased levels in some types of cancer including lung cancer, which allows these cells to grow and divide uncontrollably.2
These cancer cells can be hindered from growing by blocking the EGFR using EGFR-TKI, making this therapy a first-line standard of care.3, 4
Acquired EGFR T790M mutation
However, patients treated with EGFR-TKIs inevitably develop acquired resistance and tumor progression due to secondary mutation, EGFR T790M, after approximately 9-13 months.3, 5, 6, 7 The EGFR mutation-positive NSCLC, occurs in 30-40% of patients in Asia.8
The T790M mutation is an acquired resistance that produces a drug-resistant variant of the targeted kinase which confers growth advantage and survival of lung cancer cells. This mutation is present in about half of lung cancer patients following first- or second- generation EGFR-TKI therapy. 9, 10, 11
Second-line Treatment
As mutational subclones may evolve over time, liquid biopsy allows for real-time monitoring of clonal changes to guide the second-line treatment in patients and to determine T790M mutation status to maximize the number of patients who are able to receive subsequent treatment with the third-generation EGFR TKI. 11, 12
Future Treatment Paradigm
Other exploratory strategies (e.g., bypass pathways, downstream signaling, histologic transformation, and others) are under development to identify patients that are suitable for molecular targeted therapy to overcome the resistance mechanisms which may change the future treatment paradigm based on preclinical and clinical studies.11
At Pantai Premier Pathology, we provide Lung Cancer Tests:
- EGFR Mutation Testing (Tissue)
- EGFR T790M mutation Liquid Biopsy Testing
- 170 Genes Next Generation Sequencing Testing
- 523 Genes Next Generation Sequencing with Tumour Mutational Burden Testing
For more information on the tests provided, please contact us at +603-42809115 (Customer Service) or email us at info@premierpathology.com.my
References
1- Key Statistics for Lung Cancer. (n.d.). American Cancer Society. Retrieved August 2, 2021, from https://www.cancer.org/cancer/lung-cancer/about/key-statistics.html
2- epidermal growth factor receptor tyrosine kinase inhibitor. (n.d.). National Cancer Institute (NIH). Retrieved August 2, 2021, from https://www.cancer.gov/publications/dictionaries/cancer-terms/def/epidermal-growth-factor-receptor-tyrosine-kinase-inhibitor
3- Bursac, D., Zarić, B., Kovačević, T., Stojšić, V., Vagionas, A., Boukovinas, I., … & Sekerus, V. (2021). Personalized Approach to Tissue and Liquid Biopsy after Failure of First-Line EGFR-TKIs: Is There an Issue When Tissue Is Not the Issue? A Case Series. Case Reports in Oncology, 14(2), 716-724.
4- Planchard, D., Boyer, M., Lee, J. S., Dechaphunkul, A., Cheema, P., Takahashi, T., … & Ohe, Y. (2018). 128O Osimertinib vs standard of care (SoC) EGFR-TKI as first-line therapy in patients (pts) with untreated EGFRm advanced NSCLC: FLAURA post-progression outcomes. Journal of Thoracic Oncology, 13(4), S72-S73.
5- Helena, A. Y., Arcila, M. E., Rekhtman, N., Sima, C. S., Zakowski, M. F., Pao, W., … & Riely, G. J. (2013). Analysis of tumor specimens at the time of acquired resistance to EGFR-TKI therapy in 155 patients with EGFR-mutant lung cancers. Clinical cancer research, 19(8), 2240-2247.
6- Suda, K., Onozato, R., Yatabe, Y., & Mitsudomi, T. (2009). EGFR T790M mutation: a double role in lung cancer cell survival?. Journal of Thoracic Oncology, 4(1), 1-4.
7- Wu, W. S., & Chen, Y. M. (2014). Re-Treatment with EGFR-TKIs in NSCLC Patients Who Developed Acquired Resistance. Journal of personalized medicine, 4(3), 297–310. https://doi.org/10.3390/jpm4030297
8- Ellison, G., Zhu, G., Moulis, A., Dearden, S., Speake, G., & McCormack, R. (2013). EGFR mutation testing in lung cancer: a review of available methods and their use for analysis of tumour tissue and cytology samples. Journal of clinical pathology, 66(2), 79–89. https://doi.org/10.1136/jclinpath-2012-201194
9- Suda, K., Onozato, R., Yatabe, Y., & Mitsudomi, T. (2009). EGFR T790M mutation: a double role in lung cancer cell survival?. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer, 4(1), 1–4. https://doi.org/10.1097/JTO.0b013e3181913c9f
10- Choo, J. R., Tan, C. S., & Soo, R. A. (2018). Treatment of EGFR T790M-Positive Non-Small Cell Lung Cancer. Targeted oncology, 13(2), 141–156. https://doi.org/10.1007/s11523-018-0554-5
11- Liao, B. C., Griesing, S., & Yang, J. C. (2019). Second-line treatment of EGFR T790M-negative non-small cell lung cancer patients. Therapeutic advances in medical oncology, 11, 1758835919890286. https://doi.org/10.1177/1758835919890286
12- Hochmair, M. J., Buder, A., Schwab, S., Burghuber, O. C., Prosch, H., Hilbe, W., Cseh, A., Fritz, R., & Filipits, M. (2019). Liquid-Biopsy-Based Identification of EGFR T790M Mutation-Mediated Resistance to Afatinib Treatment in Patients with Advanced EGFR Mutation-Positive NSCLC, and Subsequent Response to Osimertinib. Targeted oncology, 14(1), 75–83. https://doi.org/10.1007/s11523-018-0612-z